Developing MRI Protocols for Brain Imaging of the Critically Endangered Smalltooth Sawfish
The smalltooth sawfish (Pristis pectinata) is one of the most evolutionarily distinct and ecologically threatened elasmobranchs. Understanding its brain and sensory organisation can provide insight into how this species perceives and interacts with its environment (check out our previous blog post to see how!). Our project aims to develop and optimise magnetic resonance imaging (MRI) protocols to non-destructively characterise the neuroanatomy of P. pectinata.
Using MRI to study endangered species
MRI allows high-resolution, non-invasive, and three-dimensional visualisation of soft tissue structures. It works by aligning the hydrogen protons in water molecules using a strong magnetic field, then detecting how those protons respond to radiofrequency pulses. The resulting images can be digitally reconstructed to quantify the volume of different structures, such as brain regions or sensory organs.
Because P. pectinata is critically endangered, preserving all available material is essential. MRI provides a way to examine nervous system morphology while maintaining the integrity of valuable specimens for future analyses with collaborators.
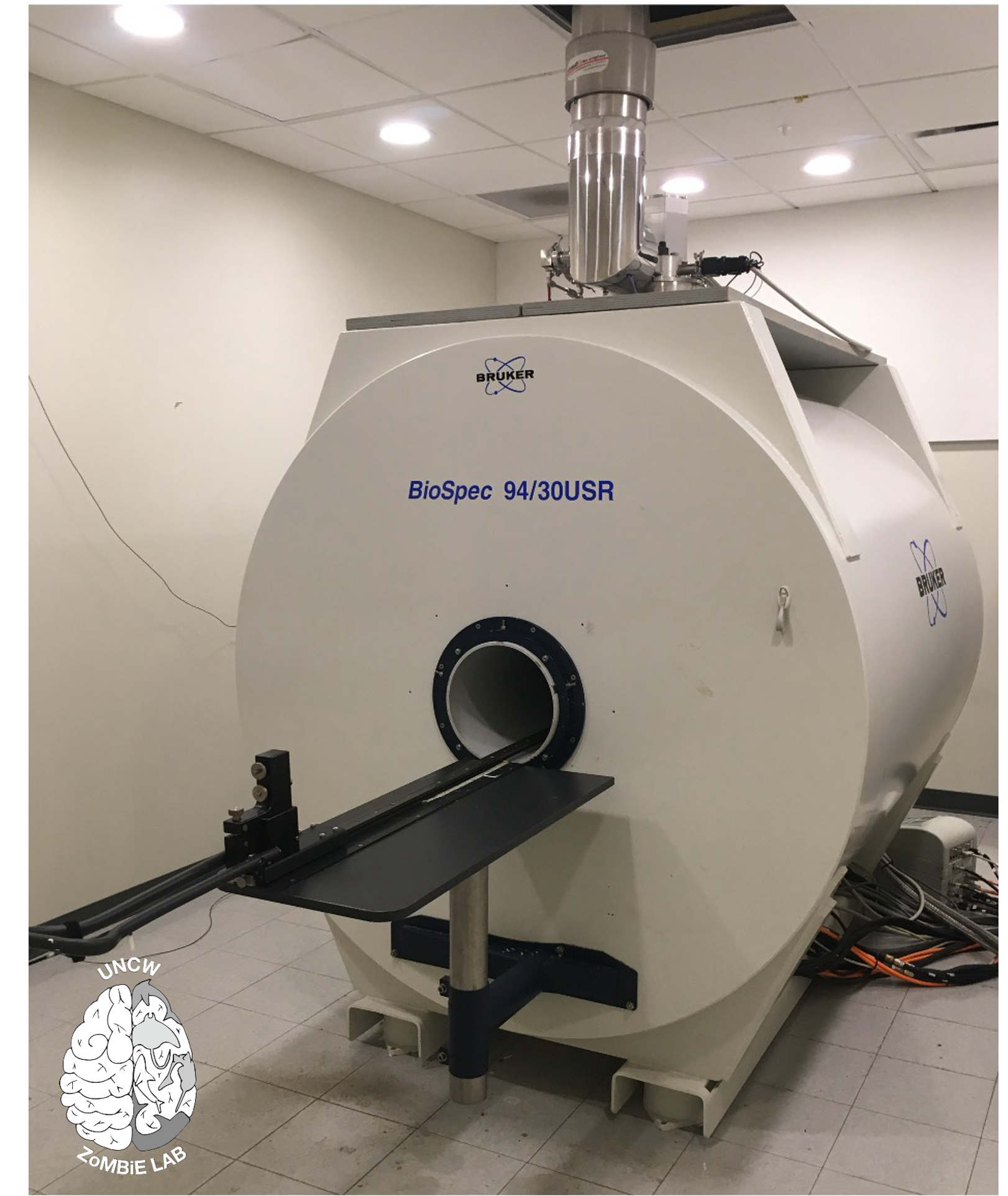
9.4 Tesla animal scanner at the Biomedical Research Imaging Center, UNC Chapel Hill. Photo © E. Peele
Scaling up from small to large samples
ZoMBiE Lab Member Emily Peele focused her dissertation work on neuroplasticity in elasmobranch fishes, examining how environmental factors such as temperature affect brain development and organisation. Much her work involved small embryos and juvenile elasmobranchs measuring 5–20 cm in length. By comparison, adult P. pectinata can reach up to five meters and several hundred kilograms. Their brains are correspondingly large and require a very different approach to imaging.
In Emily’s work, whole specimens could be imaged intact within standard MRI chambers. In contrast, the large size of P. pectinata requires removal of the brain prior to scanning. This process is considerably more complex due to the size and density of cranial structures, which requires power tools for dissection rather than the surgical instruments typically used for smaller specimens.
Optimising MRI parameters for large tissue
We are conducting scans on both a 9.4 Tesla animal scanner and a 7 Tesla human scanner at the Biomedical Research Imaging Center (BRIC) at the University of North Carolina at Chapel Hill (Fig. 1). While the general workflow is similar to previous imaging (embedding samples in a proton-free media to eliminate air and motion artifacts), the larger tissue volume requires extended preparation times. Fixatives must be thoroughly removed from the brains before contrast soaking, and the gadolinium-based contrast used to enhance tissue differentiation requires longer diffusion periods.
MRI systems are also highly sensitive to metal interference, so all imaging chambers must be completely non-metallic. The UNC Physics Department built three custom, metal-free chambers for this project. These allow us to safely position and stabilise large samples within the magnet while maintaining image quality.
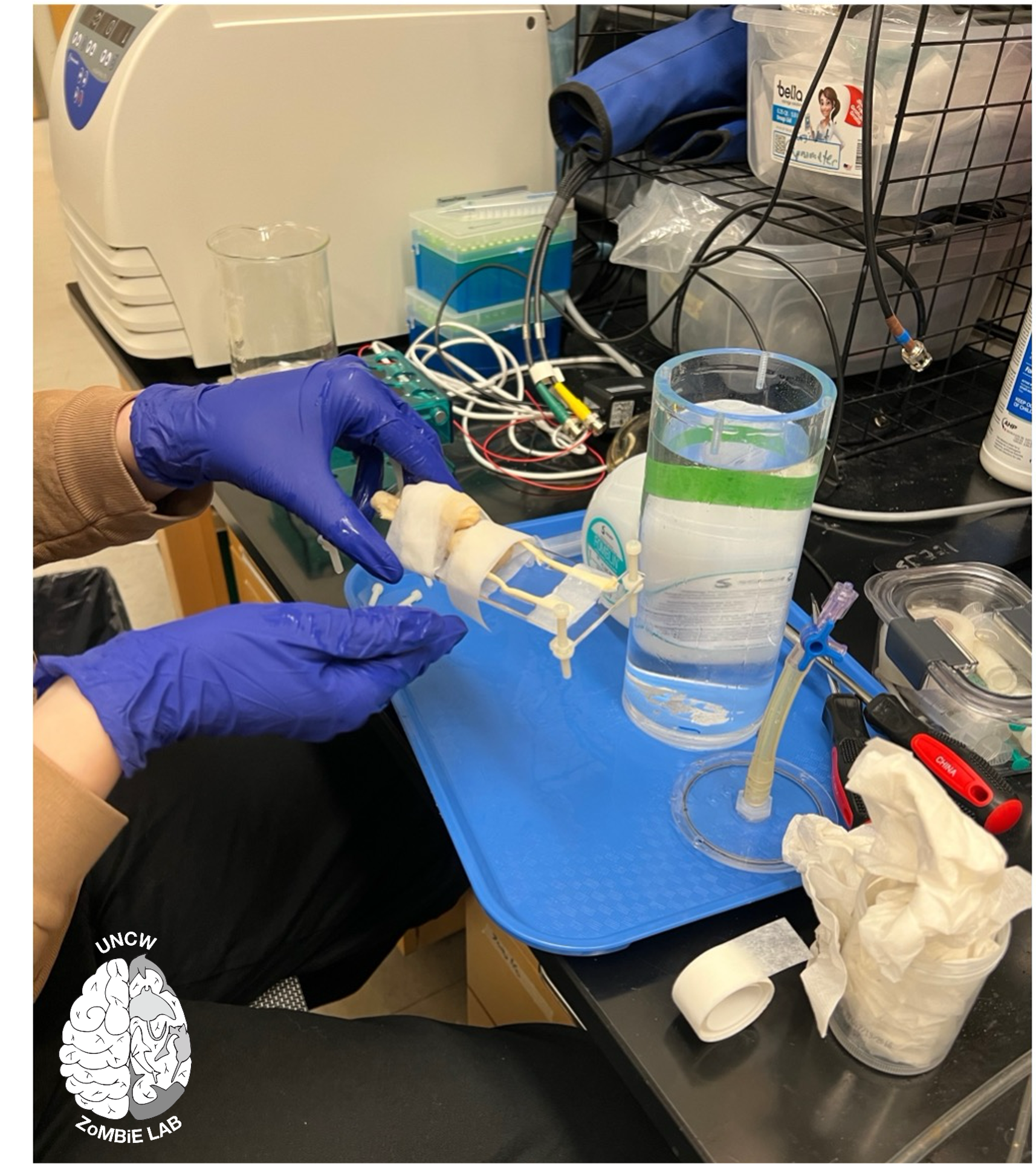
Preparing a sawfish brain for MR scanning at BRIC within a custom-built imaging chamber. Photo © E. Peele
Optimisation for this project has focused on adjusting scan duration, field of view, and contrast parameters to achieve consistent resolution across the entire sample. The first complete datasets verified that the optimised parameters produced consistent, high-resolution images of the major brain regions.
Next steps
These scans will serve as a baseline for comparing brain morphology across batoids and throughout ontogeny in P. pectinata. They will also support future analyses of neuroanatomical variation in individuals from recent mortality events in South Florida. This project represents one of the first applications of high-field MRI to sawfish neuroanatomy and establishes a foundation for future comparative and conservation-focused studies of the elasmobranch nervous system.
A project such as this requires collaboration across a massive team of amazing researchers. In addition to support from SOSF, this work is bringing together FWC, the Fish and Wildlife Foundation of Florida, Havenworth Coastal Conservation, and the Bonefish Tarpon Trust.
Want to read more about some of our lab’s work we discussed above? Check out our publications page!
