Turning careful planning into results!
In previous blog posts, we established that environmental DNA (eDNA) research is more than simply taking water samples and magically finding out if your target species was detected. Being sure that your team is using the latest methods and taking steps to avoid false negatives and false positives are key, especially when conducting research on rare species like sawfishes that require special management and conservation actions. The goal of this post is to share how all that planning has turned into some preliminary results!

A Smalltooth Sawfish entangled in fishing gear off the coast of Florida. Photo © Michael Patrick O'Neill
We are studying the Critically Endangered Smalltooth Sawfish, which is currently found in less than 20% of its former range; viable populations are confined to the U.S. and the Bahamas. We wanted to explore eDNA as a potential tool that could be used for monitoring recovery of this species in the western Atlantic Ocean to aid in prioritizing where to focus future research and education efforts.
First, we need to define 2 terms. The current U.S. Smalltooth Sawfish population is restricted to the south and southwest Florida, which is referred to as the core range by recovery planners. Anything outside this core range is considered the non-core range and will be looked at as potential areas for recovery.
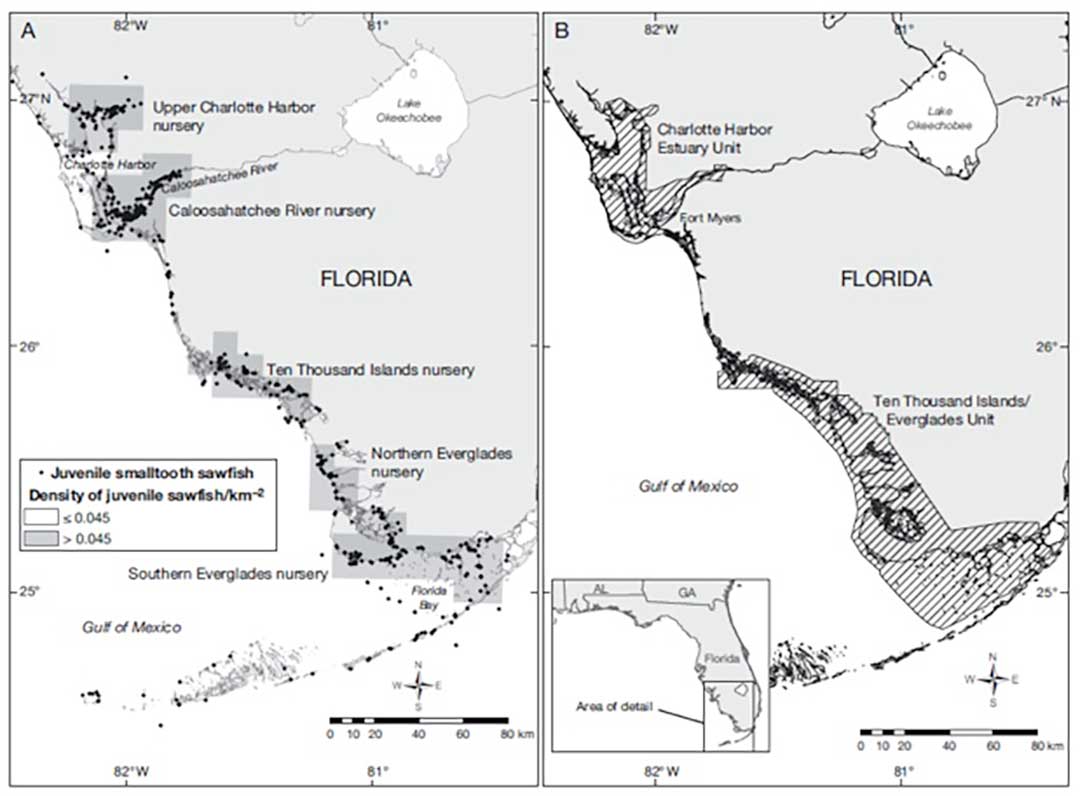
(A) Five Smalltooth Sawfish nursery areas identified in southwest, Florida, USA, and (B) 2 designated juvenile Smalltooth Sawfish critical habitat units, modified from Norton et al. (2012); figure taken from Brame et al. (2019). This portion of Florida is considered the current core range of the Smalltooth Sawfish in the USA.
For this project, we chose the Indian River Lagoon on Florida’s east coast and Tampa Bay on the west coast to focus our eDNA sampling. On the basis of what we’ve learned in recent years about habitat use by juveniles and temperature sensitivity of the species, these estuaries are likely candidates for range expansion and new nursery establishment if the species begins recovering.
The Indian River Lagoon stretches along about one-third of the east coast of Florida and historical scientific literature suggests that this estuary was once a thriving nursery for the Smalltooth Sawfish. Currently, the southern portion of the lagoon has the habitats and temperature regime to support the species, so we targeted this area for sampling. Of the 30 water samples we collected in 2018, 4 were positive for Smalltooth Sawfish DNA! We can’t identify the life history stage of the individuals from the eDNA, but since they were well within the estuary, there’s a good chance they were immature since adults tend to be found in the ocean along the beaches or even further offshore.
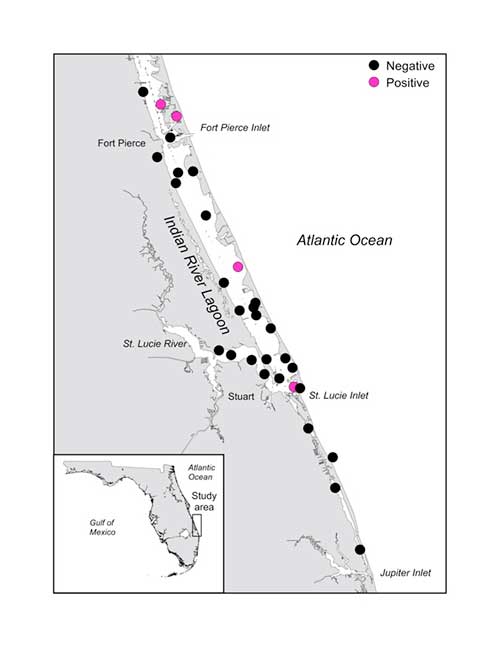
Locations and results of 30 water samples taken in July 2018 in the Indian River Lagoon, Florida to look for Smalltooth Sawfish eDNA. Artwork © Rachel Scharer.
Tampa Bay is the next major estuary to the north of Charlotte Harbor, which is located at the northern limit of the current core range of the species on Florida’s west coast. We targeted the western portion of this estuary near habitats likely to support juvenile sawfish. Of the 20 water samples analyzed so far, 3 were positive for Smalltooth Sawfish! Again, we don’t know the life history stage of the individuals that were detected, but since they were well within the estuary, including 2 in the Little Manatee River, there’s a good chance they were immature. These data have already been used by another Save Our Seas project leader, Tonya Wiley, as 1 line of evidence to help select sample sites for tagging Smalltooth Sawfish in this estuary.
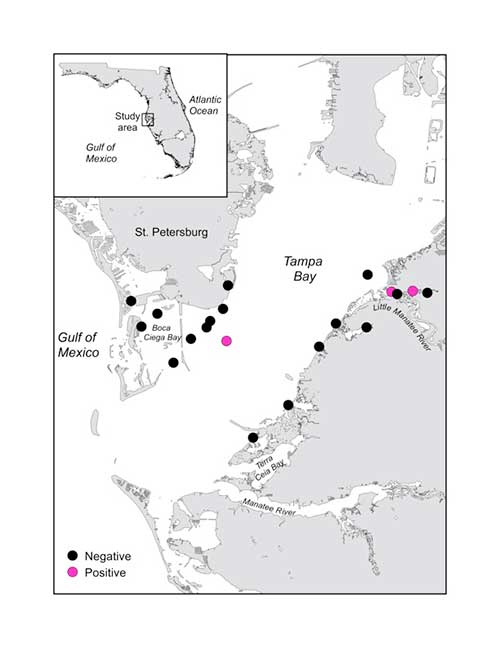
Locations and results of 20 water samples taken in August 2018 in Tampa Bay, Florida to look for Smalltooth Sawfish eDNA. Artwork © Rachel Scharer.
So, we’re well on our way to showing that our environmental DNA tool can be used successfully in the western Atlantic. The methods have been developed and validated in our part of the world, but our techniques can be customized for other regions, so please reach out if you’re considering using this tool elsewhere. We’ll be happy to help!
Special thanks to Rachel Scharer, Ryan Lehman, Alia Court, John Hadden, and Andrew Wooley for assistance in the field and with filtering samples. Thanks to Nicole Phillips and Ryan Lehman for droplet digital PCR sample processing. That’s it for now but look for future blogs that will contain more updates on our progress.
