Haematology and Hormones: How to move from sample collection to data!
Our last blog focused on collecting samples from tope sharks in Pen Llŷn a’r Sarnau Special Area of Conservation during the 2025 field season for our project ‘Where do tope sharks mate and pup?’. But once our samples are collected how do we use them to understand whether tope are using Pen Llŷn a’r Sarnau SAC to mate or pup?
Our main goal in 2025 (alongside tagging sharks with acoustic transmitters to investigate movements and habitat use) was to collect blood samples from tope which we can then analyse to quantify concentrations of reproductive hormone. However, getting samples from a shark on a boat in the Irish Sea to a lab for analysis is trickier than it sounds – involving careful coordination, sample processing and storage, and collaboration across several institutions!
Sample processing and storage
We collect small blood samples (~2-3 mL) from tope via the caudal vein that runs along the spine. Once the blood is collected it is immediately transferred to a tube which is pre-treated with heparin to prevent blood from clotting in the sample tube. We then store samples on ice until we return to dock at the end of fishing trips.
Once back at our base, samples are processed for long-term storage. This involves measuring haematocrit (the percentage of blood volume comprised by red blood cells) before centrifuging blood to separate red and white blood cells from the plasma. We then freeze the samples at -80 °C until the end of the field season when we shipped them to Dr Kirsten Wilson, a biomolecular assay specialist at the University of Edinburgh to analyse.
Sample analysis
Once samples arrive in Edinburgh, Kirsten uses Enzyme Linked Immunosorbent Assays (ELISA’s) to analyse levels of 17β-estradiol, Testosterone, Progesterone, and Thyroid hormone in plasma from tope. During an ELISA, a small amount of plasma is added to the well of a microplate which contains antibodies which bind to the hormone of interest. The amount of antibodies that are bound vs unbound depends on the concentration of hormone in the sample.
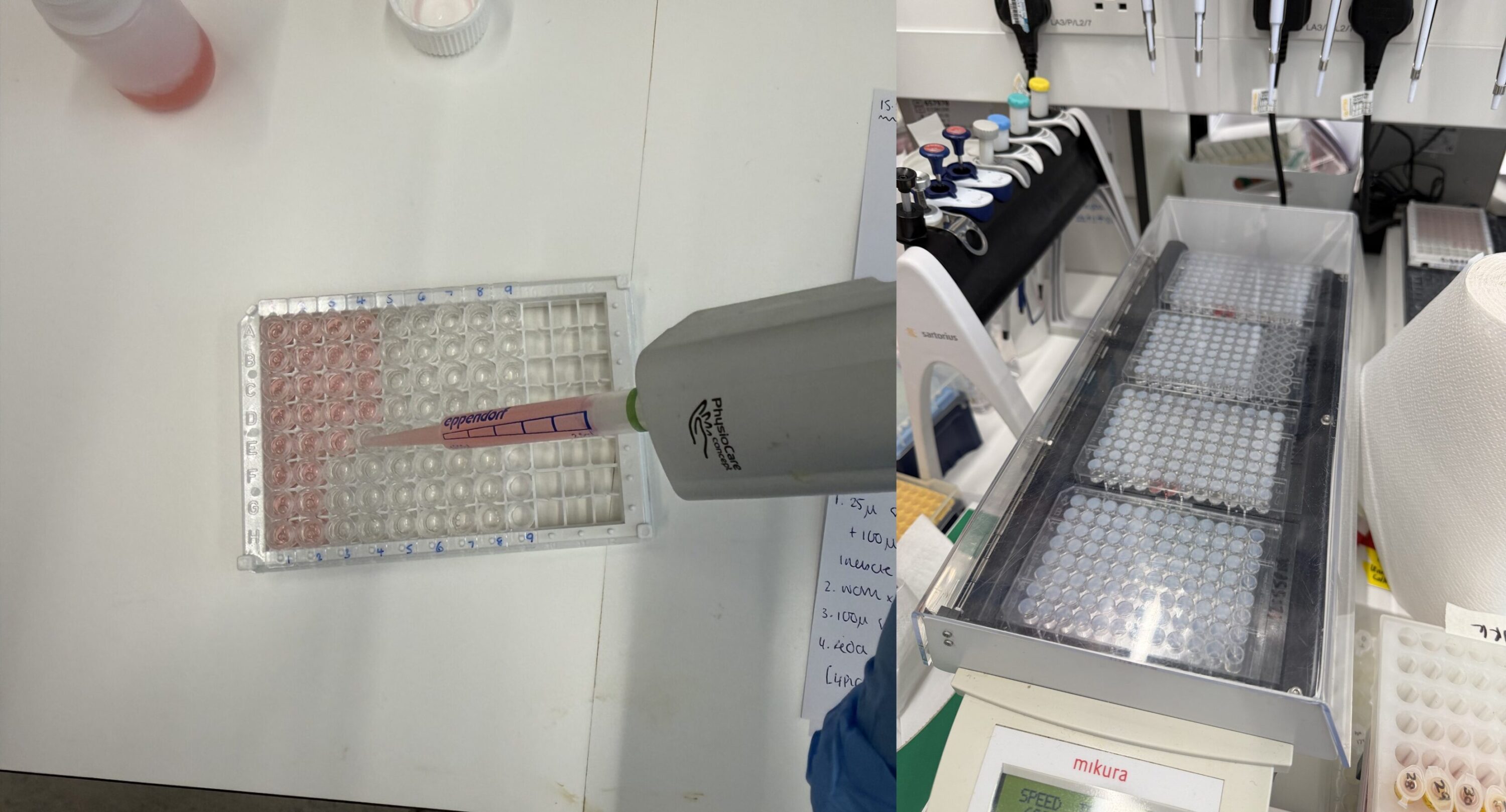
Ar y chwith: Ychwanegir sampl plasma ac adweithydd at bantiau mewn microplât. Ar y dde: caiff y plât ei ddeori ar 28 °C am ddwy awr i ganiatáu i’r hormonau a’r antigenau sy’n gysylltiedig ag ensymau rwymo’n gystadleuol. Lluniau © Kirsten Wilson
A colourless substrate is then added to the plate which, over time, reacts with the bound antibodies in the plate to produce a blue colour. The intensity of the colour produced by the reaction is dependent on the amount of hormone-bound antibodies – which is itself inversely related to the concentration of hormone in the original sample. At the end of the incubation the reaction is stopped turning the colour from blue to yellow.

Ar y chwith: Ar ôl ei olchi, ychwanegir swbstrad (o’r enw TMB). Mae hwn yn ddi-liw ond mae’n troi’n las dros amser. Mae dwyster y lliw yn dibynnu ar faint o hormon sy’n bresennol yn y sampl. Ar y dde: Ar ôl 10 munud, ychwanegir toddiant ‘stop’ asidig sy’n gwneud i’r lliw droi’n felyn ar unwaith. Lluniau © Kirsten Wilson
Finally, the transmission of light at a set wavelength through the sample can be measured. Samples with a stronger colour will allow less light through them which allows Kirsten to quantify the hormone levels from our samples by comparing light transmission through our samples (which have unknown hormone levels) with light transmission through standards in which a known concentration of hormone is added.
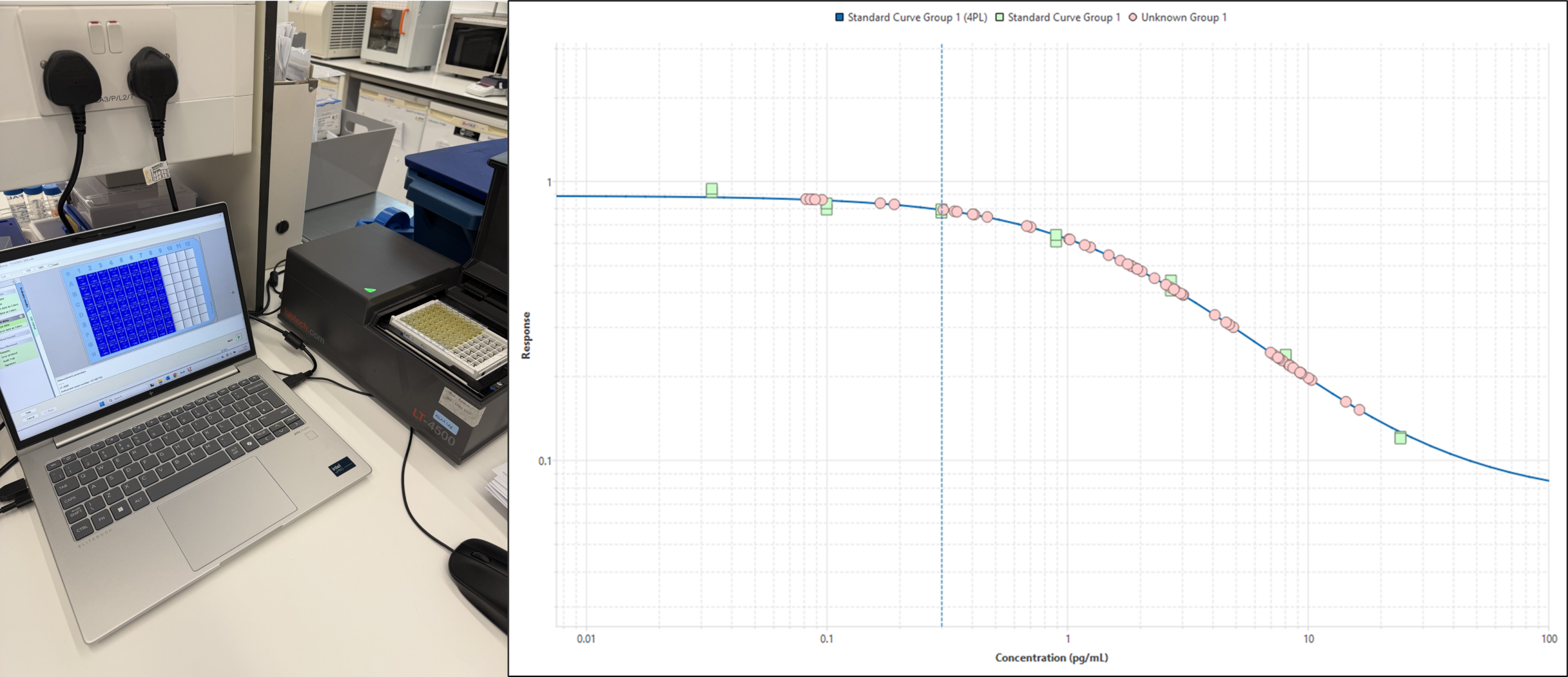
Ar y chwith: Mae trosglwyddiad golau trwy bob twll yn y microplât yn cael ei fesur ar sbectromedr plât. Ar y dde: Gan ddefnyddio rheolyddion crynodiad hormonau hysbys (sgwariau gwyrdd) crëir cromlin safonol (llinell las) y gellir ei defnyddio wedyn i gyfrifo’r crynodiadau hormonau ym mhob sampl (cylchoedd pinc). Lluniau © Kisten Wilson
Now we have this data we can start to work out whether the tope we sampled months earlier show signs that they were reproductively active or pregnant when they were caught!
