Finding New Solutions to Old Problems
Last year when I was writing the proposal for my research project, I stumbled upon the novel idea of using dried blood spot analysis as an analytical technique for estimating the trophic level for my targeted shark species. Trophic levels can be defined as the position an individual occupies within the food chain ranging from primary producers (phytoplankton, seagrass) to apex predators (large shark species, tunas, dolphins). Understanding a species’ trophic level within a marine environment tells a story about the interrelationships between species (predator and prey), but also helps us to understand how energy is transferred throughout an ecosystem. To determine the trophic levels at which a consumer is feeding we collect biological samples such as blood or muscle tissue to assess the level of naturally occurring isotopes like nitrogen (15N). Nitrogen levels give us a relatively good estimate of trophic level because these isotopes accumulate in larger quantities the higher an individual is within the food chain. Naturally, I thought stable isotope analysis would pair well with my movement ecology research. However, dried blood spot analysis has not been implemented with shark species before.
You may be thinking to yourself, “Why are we using a new stable isotope technique for elasmobranchs if we already have reliable methods that work?” Before I started this project, I would have asked myself the same question, but the logistical challenges with collecting samples at the Flower Garden Banks National Marine Sanctuary meant we had to find an easier and more efficient solution to estimate the trophic ecology of shark species in this region. The complications of working at an isolated system in the Gulf of Mexico meant the elevated ambient temperatures during the summer and distance from the mainland (> 160 km from shore) made it difficult to properly preserve and transport biological samples back to the university in a timely manner.

Collecting a blood sample from a juvenile silky shark via the caudal vein. This sample will be spun in a centrifuge to separate whole blood into plasma and red blood cells which will be stored separately. Photo © Brett Sweezey
To overcome these challenges, our team decided to implement dried blood spot analysis to estimate the trophic levels of three different shark species within the Flower Garden Banks sanctuary. The sandbar (Carcharhinus plumbeus), silky (Carcharhinus falciformis), and scalloped hammerhead (Sphyrna lewini) are three shark species resident to this region of the Gulf of Mexico which each occupy a separate ecological niche. To create a dried blood spot, a sample of blood is extracted from the caudal vein at the base of the caudal fin and a small volume of blood is applied and dried onto cellulose paper. This technique provides an efficient method to collect, transport, and store blood samples at ambient temperatures without the stress of needing to preserve samples in the field until they can be moved to a long-term storage facility back at the lab. Traditionally these tests have been on humans to test antibodies, antigens, or nucleic acids, but dried blood spots have gained traction as a nonlethal sampling technique in ecology studies because of their low-cost sampling and this technique has not yet been applied to elasmobranch species.

A blood sample collected by a syringe is placed into a vacutainer to reduce the chance of blood coagulation during the transportation and storage process back to the lab where it will be separated for long term storage. Photo © Brett Sweezey
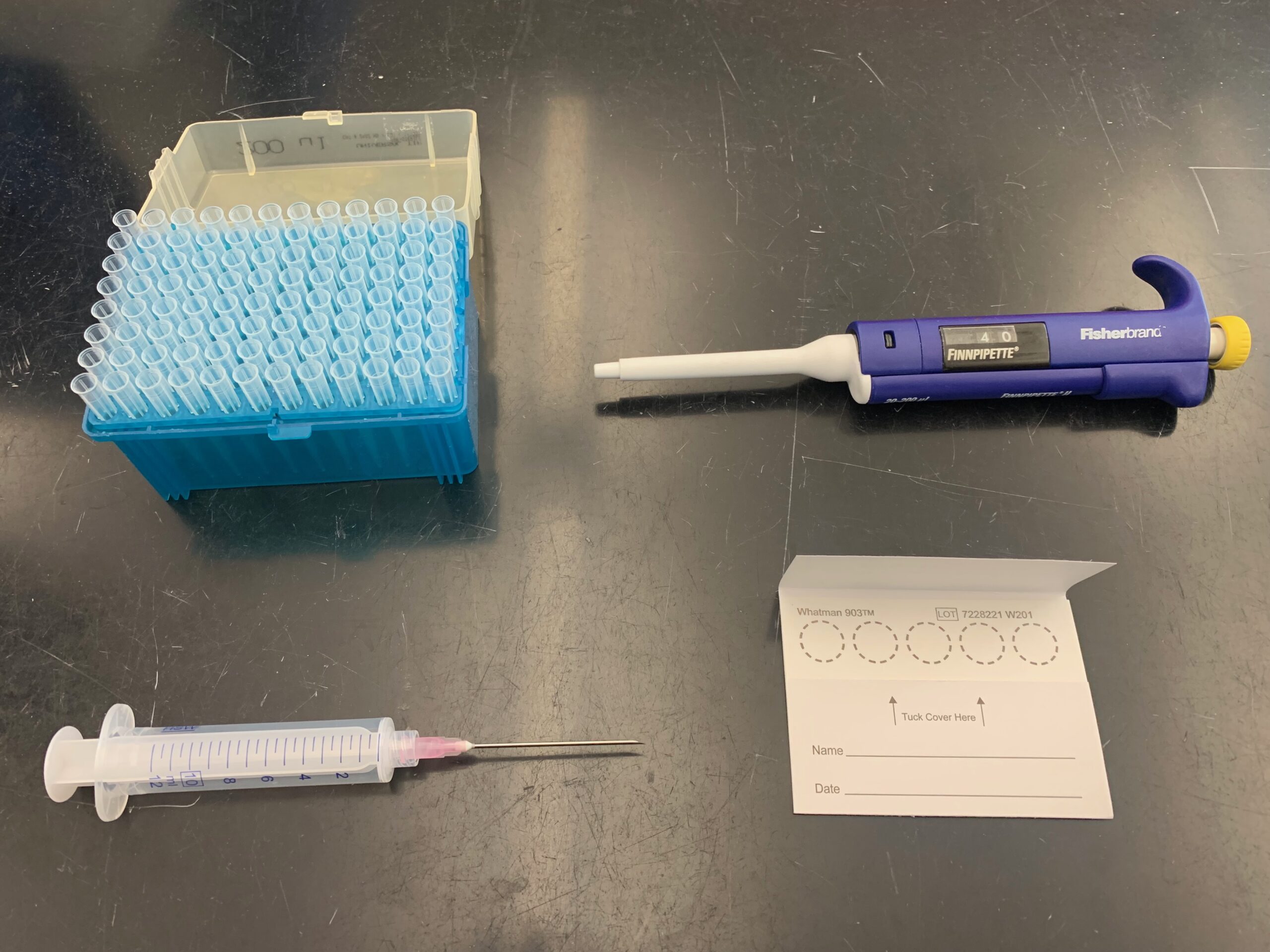
All the materials needed to collect and create a dried blood spot. Pipette tips (upper-left), micropipette (upper-right), needle and syringe (bottom-left), and cellulose card (bottom-right). Photo © Brett Sweezey
Our goal is to test the efficiency of dried blood spot analysis against traditional stable isotope methodologies by comparing the nitrogen levels from dried blood spot samples against the nitrogen levels observed in the muscle tissue, whole blood, plasma, and red blood cells of the same individual. If this research is effective, stable isotope techniques which utilize more invasive sampling techniques such as muscle extraction can be substituted for dried blood spot methodology to expand our access of sampling in resource-limited settings, reduce logistical and financial challenges associated with stable isotope analysis, and increase our understanding of trophic ecology in areas of high ecological importance.

Completed and ready to be stored dried blood spot samples. Photo © Brett Sweezey
Stay tuned for more updates as we continue our journey to collect and analyze these samples!
